Certain chemical elements are responsible for body color in varying gem materials. Chromium is a chromophore responsible for the bright red of ruby and the bright green of emerald (Douma, 2017). The type, number, and arrangement of atoms in gemstones can affect what our brain interprets as color. It is very common for the same transition elements in gemstones to produce differing effects in different environments (Gem-A, 2009).
Ruby
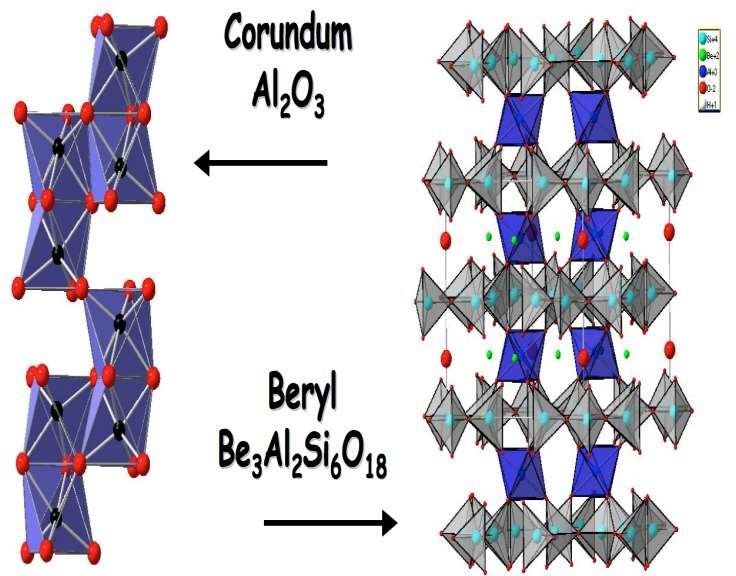
The rich red color of ruby, Al2O3 , is due to an impurity within the aluminum oxide crystal structure. Traces of chromium (Cr3+), replace aluminum (Al3+), about one atom of aluminum per hundred is replaced by a chromium in ruby (Sicree,2007).
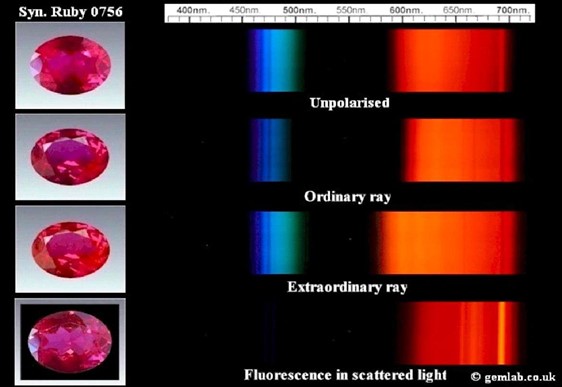
There is a broad absorption band in the green to yellow that diminishes the transmission of green to yellow light and results in the transmission of red light in ruby spectrum. Ruby has an absorption band around 550nm due to chromium.
Emerald
Emerald is a variety of the mineral beryl, Be3Al2Si6O18. The chromium ion (Cr3+) replaces aluminum (Al3+) in the mineral’s structure, very similar to that in ruby. Slight differences in the bond strengths between ruby and emerald result in shifts in the absorption and transmission bands.
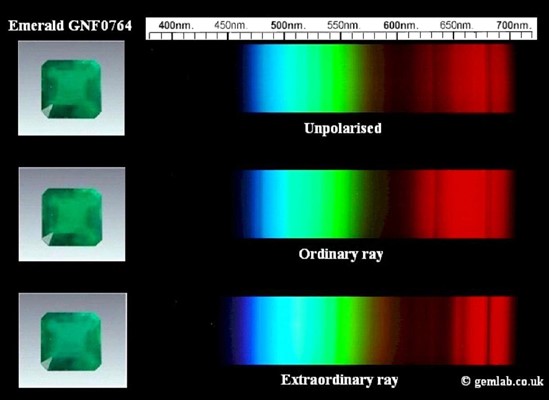
There is a slightly weaker absorption from the yellow to the red in emerald. Consequently, this sort of absorption diminishes the transmission of red light and has a resultant transmission of blue/green light. Emerald’s chromium line is around 600nm.
Alexandrite
Alexandrite and other gems that exhibit a color change effect depend delicately on the balance of the transmitted light. Daylight contains high proportions of blue and green light; while incandescent light contains a higher balance of red light. Daylight causes the stone to appear green while an incandescent light source would make the stone appear red (Gem-A, 2009 and Fritsch, and Rossman, 1987)

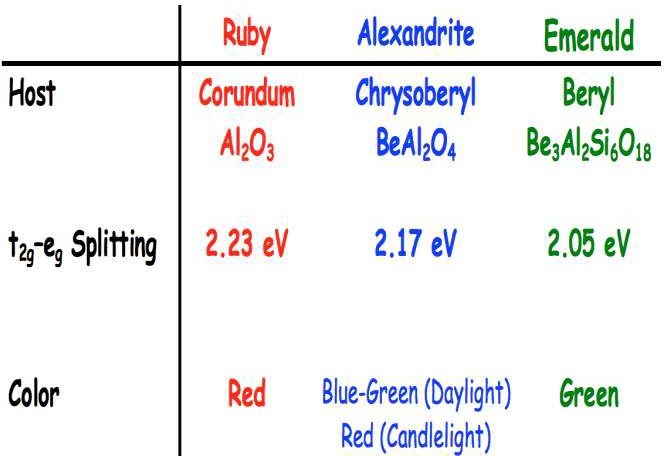
In alexandrite the chromium band is around 580nm intermediate between ruby and emerald.
Conclusion
The same number of oxygen atoms surround the Cr3+ as emerald, ruby, and alexandrite although those oxygen ions are in a differing pattern, at different distances from the Cr3+ . (Gem-A 2009) The host materials then interact with the chromium differently. When the electrons are excited (illuminated) it causes absorption of certain wavelengths of light, resulting in color. (Fritsch, and Rossman, 1987) The difference in body color can be directly related to how much energy (example. Sunlight) is required for an electron to jump from one level to another.
References
Douma, M., curator. (2008). Why are rubies red?
In Cause of Color. Retrieved 2017 http://www.webexhibits.org/causesofcolor/6AA.html
Fritsch, E., and Rossman G.R. (1987) “An Update on Color in Gems. Part 1: Introduction and Colors Caused by Dispersed Metal Ions.” Gems & Gemology Vol. 23.NO.3 https://www.gia.edu/gems-gemology/fall-1987-color-gems-fritsch
Gemmological Association of Great Britain, (2009) Diploma in Gemmology London section D7 pg 23-24
Sicree, A. A. (2007)”Chrome, Rubies, Emeralds, and Alexandrite.” Popular Mineralogy #7 http://worcestermineralclub.org/wp-content/uploads/2015/03/PopMin-07a.pdf
https://cbc-wb01x.chemistry.ohio- state.edu/~woodward/ch754/lect2003/optical1_lect21.pdf



